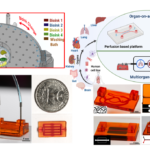
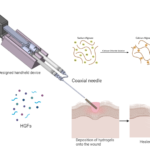
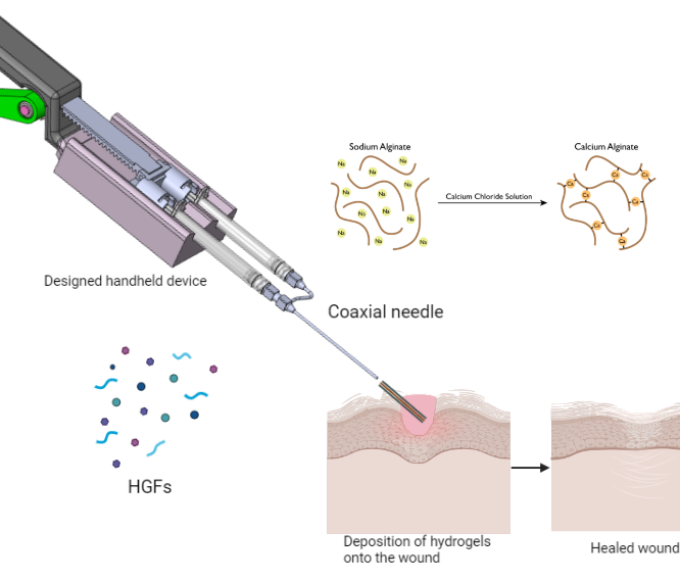
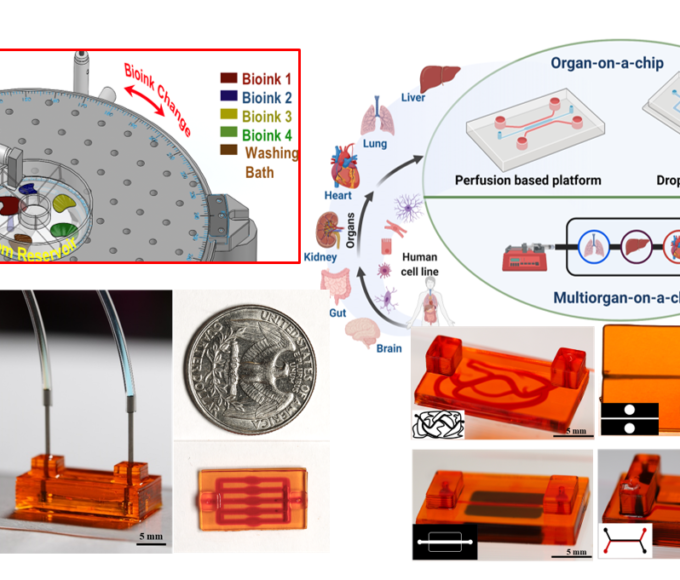
Soft-tissue sarcoma (STS) is a group of aggressive tumors that grow in non-adherent conditions and are highly invasive and resistant to chemotherapy. The low effectiveness of chemotherapy demands proper pre-clinical STS models to evaluate anti-cancer drugs. However, there is a lack of in vitro models recapitulating tumor microenvironment (TME) within STS. Therefore, there is a critical need for better organotypic drug screening platforms to improve systematic therapies, reduce fabrication time, and identify predictive factors for response to chemotherapy drugs. The long-term goal of this research is to design and develop a bioprinted vascularized STS tumor spheroid-on-a-chip that will lead to possibilities for cost-effective drug discovery and personalized medicine. We have shown that multimaterial bioprinting has allowed us to tailor the mechanical properties of ECM mimicking hydrogel and positioning biologics on desired locations. This further will allow us to recapitulate the TME of soft tissue tumors and monitor cell behavior. Our published data and new preliminary data support the relevance of this model provide the basis for our hypothesis that the construction of an in vitro model can mimic the cellular composition and the ECM properties of tumor tissue for its in vivo counterparts. The immadiate goal of the proposed studies is to provide a proof-of-concept of bioprinted tumor-on-a-chip to meet the clinical need for rapid and inexpensive 3D in vitro soft tissue tumor models and drug screening platforms. Two specific aims are proposed 1) validating the biomimicry of our bioprinted tumor model and 2) measuring the specific cell markers in this organotypic tumor model. At the completion of the aims, we expect that this novel hydrogel microfluidic platform will provide a valuable tool for invasion with sarcoma spheroids used for drug discovery and development.